Thalamic Glioma: Insights into Diagnosis and Treatment
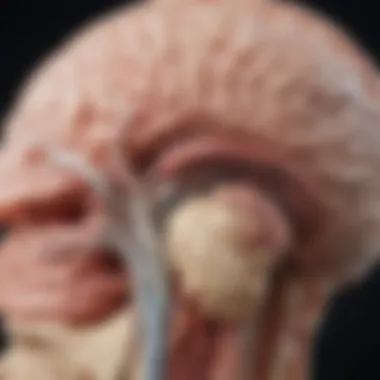
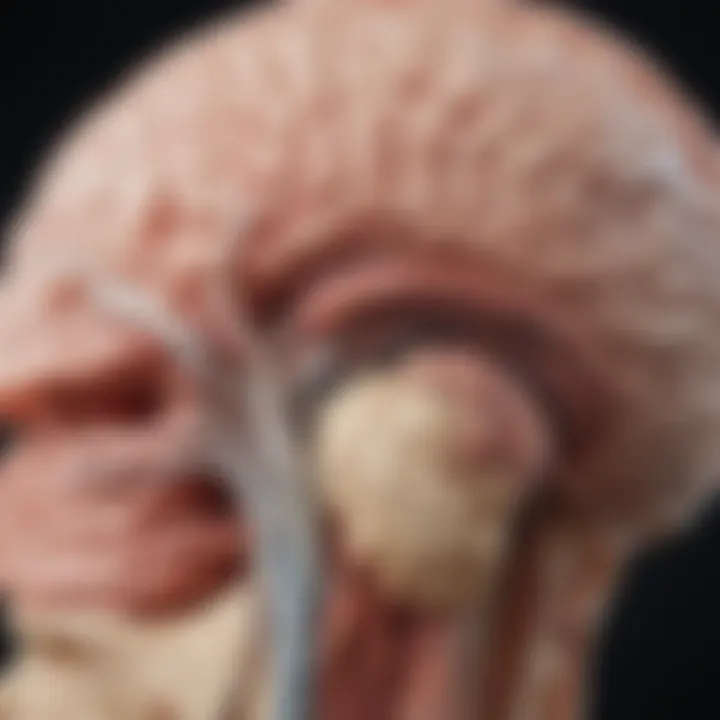
Background and Context
Thalamic gliomas are a rare group of brain tumors that occur in the thalamic region of the brain. This area plays a crucial role in sensory perception and regulation of motor functions. Understanding thalamic gliomas requires exploration of various dimensions, including their pathophysiology, clinical manifestation, and management strategies.
Historically, these tumors were not well characterized, partly due to their infrequency and complex nature. The classification of thalamic gliomas has evolved over the years, moving from vague descriptions to more precise categorizations based on histological features and molecular genetic profiles. This shift in understanding continues to inform both diagnostic and therapeutic approaches.
Key Findings and Discussion
Research has identified several key findings regarding thalamic gliomas. They can be classified primarily into two types: low-grade and high-grade tumors. Low-grade gliomas tend to have a better prognosis, whereas high-grade gliomas present more aggressive behavior and poorer outcomes.
Major Results of the Study
Clinical manifestations of thalamic gliomas vary significantly, depending on their size and exact location. Common symptoms include:
- Cognitive changes: These may involve memory issues and altered emotional responses.
- Motor deficits: Patients may experience difficulties in movement or coordination.
- Sensory disturbances: These can include changes in sensation or perception.
Neuroimaging advancements play a critical role in the diagnosis and management of thalamic gliomas. MRI and CT scans contribute valuable information about tumor size, location, and potential impact on surrounding structures. Moreover, recent advancements in genetic profiling are identifying specific mutations associated with these tumors, allowing for more targeted treatment strategies.
Detailed Analysis of Findings
The management strategies for thalamic gliomas differ based on tumor grade and individual patient factors. Surgical resection remains a cornerstone of treatment, particularly for low-grade tumors. However, high-grade gliomas often necessitate a more multimodal approach, incorporating radiotherapy and chemotherapy.
Current research is leaning towards personalized medicine, tailoring treatment plans based on individual tumor genetics.
Emerging studies suggest that understanding the molecular landscape can potentially lead to breakthroughs in therapeutic interventions. This includes the exploration of novel targets for therapy, which may improve outcomes for patients.
As research progresses, ongoing studies are expected to yield deeper insights into the complexities of thalamic gliomas. Collaboration among researchers, clinicians, and institutions will be vital in advancing this field.
Prelims to Thalamic Gliomas
Thalamic gliomas represent a distinct category of brain tumors that originate in the thalamus, a crucial relay station in the brain for sensory and motor signals. The significance of studying thalamic gliomas is underscored by their unique anatomical location and the challenges they present in terms of diagnosis and treatment. These tumors, often diagnosed in pediatric populations, exhibit less common clinical presentations compared to other brain tumors, making awareness and understanding critical for effective management.
Thalamic gliomas often come with a myriad of symptoms that can impact a patient’s cognitive and physical functionality. It is essential to grasp how these symptoms differ from other types of gliomas. Additionally, research indicates that the prognosis for patients with thalamic gliomas can be quite variable, influenced by factors like age, tumor type, and treatment approach.
The exploration of current research trends concerning thalamic gliomas also highlights the advancements in diagnostic procedures, including neuroimaging techniques like MRI, which improve accurate tumor localization, and genetic profiling that offers insights into tumor behavior. Understanding these aspects contributes to better clinical outcomes and opens avenues for innovative treatment strategies.
"The complexity of thalamic gliomas necessitates a dedicated focus on their unique characteristics, to foster advances in research and clinical practice."
The importance of understanding thalamic gliomas extends beyond mere academic interest; it resonates with real-world implications for patients and their families. By synthesizing insights from ongoing research, healthcare practitioners can better address the challenges posed by these tumors. This introduction serves as a framework for examining the multifaceted nature of thalamic gliomas, guiding the reader through subsequent sections that elaborate on their classification, pathophysiology, clinical presentation, diagnostic strategies, treatment modalities, and the future direction of research.
Overall, this exploration aims to encapsulate the current state of knowledge regarding thalamic gliomas and emphasize the importance of ongoing research to enhance both understanding and therapeutic approaches.
Understanding Gliomas
Understanding gliomas is fundamental to grasp the overall impact of thalamic gliomas. These tumors, arising from glial cells, constitute a significant portion of brain neoplasms. Their relation to thalamic gliomas emphasizes the need for precise classification and targeted treatment strategies. This section provides insights into what gliomas are, highlighting their importance in brain tumor pathology but also their epidemiological context.
Definition and Types
Gliomas are brain tumors that originate from glial cells, which are non-neuronal cells in the central nervous system. They are classified into several types based on their cell of origin, degree of malignancy, and histological characteristics. The main types include:
- Astrogliomas: Arising from astrocytes, these tumors can range from low-grade to high-grade forms like glioblastoma.
- Oligodendrogliomas: These are derived from oligodendrocytes and are known for their generally better prognosis compared to other gliomas.
- Ependymomas: Originating from ependymal cells, these tumors can occur anywhere in the brain or spinal cord.
The classification system plays a critical role in guiding treatment plans and predicting outcomes in patients. Each type demonstrates distinct biological behavior, which leads to different management approaches.
Epidemiology
The epidemiology of gliomas sheds light on their incidence, prevalence, and associated risk factors. Gliomas are relatively rare, but they make up a significant proportion of primary brain tumors.
Research indicates:
- Incidence: The annual incidence of gliomas is approximately 5 cases per 100,000 persons.
- Age Factor: Although gliomas can occur at any age, they are most frequently diagnosed in adults aged 45 to 65 years.
- Gender Disparity: There is a notable male predominance, as evidence shows that men are more likely to develop gliomas than women.
- Genetic Factors: Individuals with certain genetic predispositions have higher risks for developing these tumors. Conditions like neurofibromatosis and Li-Fraumeni syndrome exemplify this.
A deeper understanding of the epidemiological data surrounding gliomas informs clinical practices. It aids researchers and practitioners in developing strategies for earlier detection and intervention.
"Epidemiological studies provide essential insights into the characteristics and behaviors of gliomas, helping to shape future research and clinical approaches."
In summary, understanding gliomas is not only about defining their characteristics, but also about recognizing their critical role in the context of thalamic gliomas. By comprehending the different types and their epidemiological profiles, we can enhance diagnostic protocols and personalize treatment strategies, ultimately improving patient outcomes.
Anatomy of the Thalamus
Understanding the anatomy of the thalamus is critical when discussing thalamic gliomas. The thalamus serves as a central relay station for sensory and motor signals and plays a vital role in various brain functions, including consciousness, sleep, and alertness. Thus, comprehending its structure helps frame the context of gliomas that develop in this region. Thalamic gliomas can disrupt these functions, leading to significant clinical manifestations and complications.
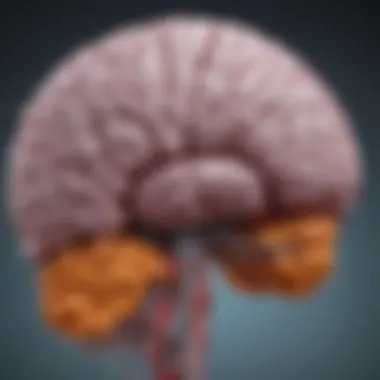
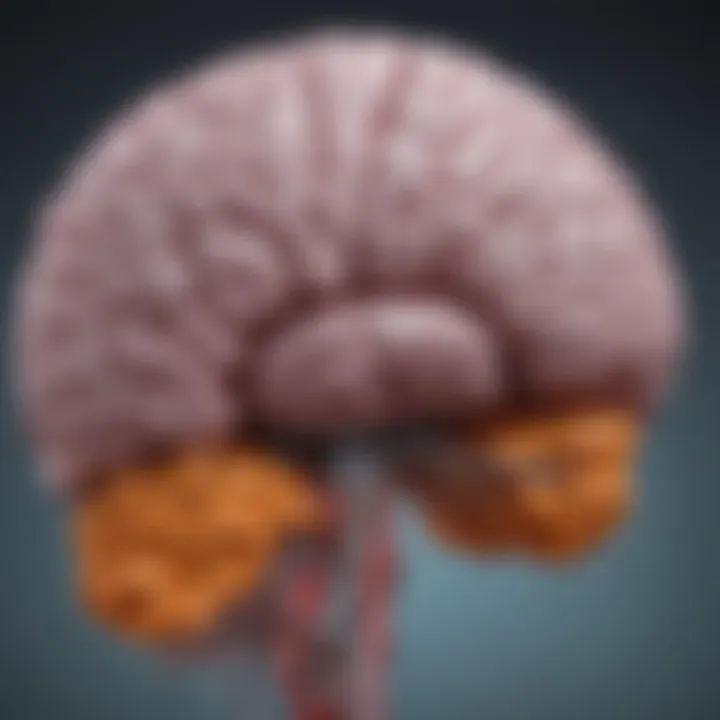
Structural Overview
The thalamus is a pair of oval-shaped structures located near the center of the brain, above the brainstem. Each thalamus is divided into several nuclei that are responsible for processing and transmitting information from different sensory modalities to appropriate areas of the cerebral cortex. The key nuclei include the lateral geniculate nucleus, responsible for vision, and the medial geniculate nucleus, crucial for auditory processing.
The thalamus itself is encapsulated by the external medullary lamina, separating it from the surrounding structures. In addition, it is surrounded by the internal capsule, which contains afferent and efferent fibers connecting the thalamus to the cerebral cortex. Understanding these structures is important because gliomas can affect not just the thalamus, but also adjacent areas, thereby compromising multiple pathways associated with sensory and motor functions.
Functional Significance
The functional significance of the thalamus cannot be overstated. It integrates and coordinates information from multiple sources, contributing to the smooth operation of complex cognitive tasks. Thalamic nuclei relay sensory information to the appropriate cortical areas. This process is crucial for sensations such as touch, taste, and proprioception. Any disruption caused by thalamic gliomas can lead to deficits in sensory perception and impact motor coordination.
Furthermore, the thalamus is involved in regulating states of sleep and wakefulness. Thalamic disturbances can result in sleep disorders, thus affecting the overall quality of life. Moreover, numerous psychiatric conditions have been associated with thalamic functioning, implicating gliomas in a broader context of mental health.
It is essential to explore the anatomy and functions of the thalamus to understand how gliomas in this area can affect not only the neurologic functions but also the psychological well-being of individuals.
In summary, the anatomy of the thalamus provides substantial insight into the implications of thalamic gliomas. Understanding its structure and functions is vital for assessing the challenges and complexities associated with these tumors.
Pathophysiology of Thalamic Gliomas
Understanding the pathophysiology of thalamic gliomas is crucial for several reasons. First, it sheds light on how these tumors develop and the biological processes that underlie their growth. This knowledge can facilitate better diagnostic approaches and treatment strategies. Thalamic gliomas manifest primarily in the thalamus, which plays significant roles in sensory perception and regulation of consciousness. Therefore, any alteration associated with these gliomas can result in varied clinical symptoms and effects on functionality.
Cellular Mechanisms
The cellular mechanisms of thalamic gliomas involve various processes that lead to tumorigenesis. At the most basic level, gliomas develop from glial cells, which are not neurons but support cells in the brain. These tumors often exhibit uncontrolled proliferation due to dysregulation in the cell cycle pathways. Key players in this process include growth factors and their corresponding receptors. The interaction between these factors can lead to enhanced cell division and survival, contributing to the aggressiveness observed in certain glioma types.
Additionally, the microenvironment of the thalamus can influence glioma behavior. Tumor-associated macrophages and the extracellular matrix play roles in shaping the tumor's growth and invasiveness. For example, gliomas can manipulate local immune responses to evade detection. This dynamic between glioma cells and the surrounding environment makes treatment challenging. Understanding these cellular interactions is vital for identifying potential targets for therapy.
Genetic Mutations Associated
Genetic mutations are pivotal in the development of thalamic gliomas. Various studies have identified common mutations found in these tumors. For instance, alterations in the genes coding for isocitrate dehydrogenase, specifically ID and ID, have been linked to glioma pathogenesis. Such mutations can affect the metabolic pathways of the tumor, leading to the accumulation of oncometabolites that drive tumor progression.
Moreover, the presence of p53 mutations is often noted in gliomas, implicating disrupted cellular survival pathways. These genetic changes not only define the malignant potential of thalamic gliomas but also can influence the response to treatment. Therapies targeting these specific alterations are currently a focus of ongoing research. Furthermore, genetic profiling can help in the stratification of patients, allowing for a more personalized treatment approach.
"Understanding genetic mutations related to thalamic gliomas can open new avenues for targeted therapies."
Classification of Thalamic Gliomas
The classification of thalamic gliomas is instrumental in guiding both diagnosis and treatment strategies. Understanding the different categories allows healthcare professionals to tailor interventions to the unique characteristics exhibited by these tumors. Accurate classification enhances prognosis forecasting and impacts decision-making. As thalamic gliomas pose significant challenges due to their location and biological behavior, an established classification system is vital to navigate complexity in clinical settings.
Grading Systems
Grading systems form the backbone of glioma classification, where tumors are categorized based on histological characteristics and their aggressiveness. The most widely accepted system is the World Health Organization (WHO) grading scale, which ranges from grade I to grade IV.
- Grade I gliomas are benign and often associated with a favorable outcome. These typically include juvenile pilocytic astrocytomas, which are more common in children.
- Grade II gliomas, such as low-grade astrocytomas, have a more infiltrative nature and present a risk for progression over time.
- Grade III consists of anaplastic gliomas, which have distinct cellular atypia and increased mitotic activity, leading to a poorer prognosis.
- Grade IV includes glioblastomas, which are highly aggressive tumors known for their rapid growth and tendency to invade surrounding brain tissue.
Understanding these grades assists clinicians in predicting tumor behavior, thus influencing treatment pathways. For example, high-grade tumors often require a more aggressive treatment approach, including surgery and adjuvant therapy, compared to low-grade tumors where observation might be more appropriate.
Histological Types
Histological classification focuses on the cellular characteristics of the tumor, which further refines treatment strategies. Several histological types of thalamic gliomas have been identified:
- Astrocytomas: These are the most common type and are characterized by the proliferation of astrocyte cells. They can range from low-grade to high-grade forms.
- Oligodendrogliomas: These tumors arise from oligodendrocytes and may present with distinct genetic markers, specifically 1p/19q codeletion, influencing treatment considerations and outcomes.
- Ependymomas: Rare in thalamic locations but still significant, ependymomas derive from ependymal cells and may exhibit different histological features depending on the growth pattern.
The histological classification, alongside the grading system, equips neurospecialists and oncologists with critical insight into tumor biology. This understanding is essential for developing personalized treatment options, including targeted therapies.
"A precise classification of thalamic gliomas is paramount for improving therapeutic outcomes and establishing targeted management strategies."
Overall, the classification of thalamic gliomas informs clinical practices, facilitates research initiatives, and ultimately contributes to advancing the field of neuro-oncology.
Clinical Presentation
Understanding the clinical presentation of thalamic gliomas is crucial for timely diagnosis and effective treatment. The symptoms can significantly impact a patient's quality of life and function. Therefore, recognizing these symptoms early can lead to faster intervention and potentially better outcomes.
Symptoms and Signs
Thalamic gliomas can manifest with a variety of symptoms. These largely depend on the tumor's size and location within the thalamus. Common signs include:
- Headaches: Often persistent and dull in nature, headaches are frequently reported.
- Neurological deficits: These may range from mild weakness to significant impairment in motor function, often affecting the opposite side of the body.
- Cognitive changes: Patients may experience difficulties with memory and executive functions. Orientation and problem-solving skills can be impacted.
- Seizures: Focal seizures are common, depending on the extent of irritation in the surrounding brain tissue.
- Sensory disturbances: Altered sensation, such as numbness or tingling, particularly can occur on one side of the body.
It is important for clinicians to conduct a thorough neurological examination if a patient presents with these symptoms. Early identification is essential due to the tumor's tendency for rapid growth and associated complications.
Impact on Functionality
The presence of thalamic gliomas can profoundly influence a person's functionality. Functional outcomes depend largely on the tumor's impact on surrounding brain structures and overall disease progression. Potential effects include:


- Compromised daily living activities: Patients may find it increasingly difficult to perform routine tasks, such as cooking or driving. Simple actions can become challenging due to motor or cognitive deficits.
- Social limitations: The psychological strain and physical limitations may lead to social withdrawal, affecting relationships and support systems.
- Occupational challenges: Employment is often impacted. Cognitive deficits can restrict career options or hinder job performance.
As noted, thalamic gliomas present unique challenges, and understanding their clinical effects is vital. Addressing these issues requires a team approach, involving neurologists, oncologists, and rehabilitation specialists.
"Early signs of thalamic gliomas can be subtle yet critical for diagnosis. Recognizing these signs is paramount."
Overall, the clinical presentation of thalamic gliomas serves as an important component of their management. Comprehensive assessments can lead to more personalized care, improving the quality of life for those affected.
Diagnostic Approaches
The diagnostic approaches to thalamic gliomas are crucial for establishing accurate and timely diagnoses. Given the complexity of these tumors and their location within the intricate landscape of the brain, proper diagnostic methods are essential for guiding treatment strategies. Successful diagnosis involves a combination of neuroimaging and pathological examination. Each method offers unique insights that contribute to a more comprehensive understanding of the tumor. In this section, we delve into the importance and implementation of these diagnostic avenues.
Neuroimaging Techniques
Neuroimaging serves as the initial step in the evaluation of thalamic gliomas. This field employs various imaging modalities to visualize the structural and functional aspects of brain tumors. The techniques commonly used include magnetic resonance imaging (MRI), computed tomography (CT) scans, and advanced functional imaging.
MRI is particularly valuable due to its ability to provide detailed images of brain tumors. High-resolution MRI can significantly aid in delineating tumor boundaries, assessing edema, and evaluating the tumor's response to treatment. Arterial spin labeling (ASL), a form of MRI, can assess cerebral blood flow, which is often affected in gliomas. Capabilities like diffusion-weighted imaging (DWI) further enhance the analysis of tumor cellularity.
CT scans, while less detailed than MRIs, are beneficial for assessing acute situations or initial evaluations, particularly in emergency settings. They can detect calcifications or hemorrhage that may influence treatment.
Additionally, positron emission tomography (PET) scans can provide metabolic information about the tumor, giving insights into its aggressiveness and potential response to therapies.
Biopsy and Pathological Examination
While neuroimaging techniques offer valuable insights, a definitive diagnosis typically requires a biopsy. A biopsy involves the removal of a small tissue sample from the tumor, which is then analyzed in a laboratory for histopathological examination. This step is essential to determine the tumor type, grade, and specific characteristics that can guide treatment.
Pathological assessment utilizes techniques such as immunohistochemistry and molecular profiling. Immunohistochemistry allows pathologists to evaluate specific markers that can indicate tumor type and behavior. This method can distinguish between different glioma subtypes, which is crucial for deciding on an appropriate treatment plan.
Molecular profiling further enhances diagnostic accuracy by identifying genetic mutations associated with thalamic gliomas, such as those found in the ID gene. These mutations not only assist in diagnosis but can also provide prognostic information and suggest potential therapeutic targets.
"Pathological examination remains the gold standard for definitive diagnosis, providing critical information on tumor characteristics, enabling a tailored approach to treatment."
Treatment Modalities
The treatment of thalamic gliomas is a critical topic in the context of this article. Thalamic gliomas represent a specific challenge in neuro-oncology due to their unusual location and the complex nature of the surrounding brain structures. Effective treatment requires a multidisciplinary approach, considering individual patient factors, tumor characteristics, and current clinical guidelines. Surgical intervention, radiation therapy, and chemotherapy are the three main modalities explored in depth below.
Surgical Intervention
Surgical intervention plays a crucial role in the management of thalamic gliomas. The aim of surgery is to achieve maximum resection of the tumor while sparing surrounding healthy tissue. This is paramount, as complete removal can improve survival outcomes and alleviate symptoms such as headaches and neurological deficits. However, the thalamus's anatomical position poses significant risks. Surgeons must navigate the risk of harming vital neural pathways that control functions such as movement, sensation, and cognition.
For these reasons, advances in neuroimaging techniques, such as functional MRI and diffusion tensor imaging, have enabled greater precision in planning surgeries. These technologies assist surgeons in mapping out critical brain areas before the operation. In cases where complete resection is not feasible, debulking surgery may still lead to symptomatic relief and improved quality of life.
Radiation Therapy
Radiation therapy is another cornerstone in the management of thalamic gliomas. It is often employed post-surgery to target residual tumor cells and reduce the risk of recurrence. The role of radiation therapy has evolved significantly, particularly with advances in techniques such as stereotactic radiosurgery and intensity-modulated radiation therapy (IMRT). These methods allow for high doses of radiation to be delivered precisely to the tumor while minimizing exposure to surrounding tissues, thus reducing potential side effects.
The timing and dosage of radiation therapy can vary based on individual patient needs, tumor grade, and overall health status. Recent studies suggest that early initiation of radiation therapy may lead to better outcomes in certain patient cohorts. However, care must be taken to balance benefits against potential complications, such as cognitive decline or radiation necrosis.
Chemotherapy Options
Chemotherapy options for thalamic gliomas are often limited, especially for high-grade tumors. The blood-brain barrier poses a considerable challenge for most chemotherapeutic agents, restricting their efficacy. Nonetheless, the use of systemic chemotherapy can be an integral part of treatment regimens, particularly for tumors that cannot be wholly resected.
Temozolomide, an oral chemotherapy drug, is commonly used for gliomas and offers some promise. Its effectiveness has been linked to the presence of specific genetic markers within the tumor. Ongoing clinical trials continue to explore various combinations of chemotherapeutic agents and inhibitors targeting molecular pathways involved in tumor growth. These investigational therapies offer hope for improved outcomes as researchers seek to develop more effective treatment modalities for thalamic gliomas.
Current Research Trends
Current research trends in thalamic gliomas are essential for advancing our understanding of this rare type of brain tumor. These trends reflect ongoing efforts to improve diagnosis, treatment, and patient outcomes. Moreover, they shed light on potential future directions that the field may pursue.
Emerging Therapeutics
Emerging therapeutics offer new hope for patients diagnosed with thalamic gliomas. Researchers are investigating a multitude of innovative treatment avenues, including targeted therapies and immunotherapy approaches. These new strategies aim to minimize collateral damage to healthy brain tissues while maximizing tumor cell destruction.
For instance, drugs that target specific genetic mutations found in tumor cells are showing promise. These medications can alter the tumor microenvironment, providing a more conducive setting for traditional treatments like chemotherapy and radiation. Researchers are also studying immune checkpoint inhibitors, which can allow a patient's immune system to better recognize and attack glioma cells.
A few notable examples of emerging therapeutics include:
- Nivolumab: This immunotherapy agent has shown some efficacy in treating gliomas, activating the immune system to fight the tumor.
- Bevacizumab: Often used in various cancers, this angiogenesis inhibitor is being explored for its potential in thalamic gliomas by impairing the tumor’s blood supply.
- Targeted therapies: Agents like IDH inhibitors are simultaneously being studied for their ability to target specific metabolic pathways associated with gliomas.
These therapeutic advancements come with considerations. Monitoring side effects, understanding resistance mechanisms, and evaluating long-term impacts on brain function remain critical areas for further study.
Clinical Trials
Clinical trials represent an essential component of current research trends in thalamic gliomas. These trials facilitate the systematic investigation of new treatments and novel combinations of existing therapies. They also provide insight into the effectiveness and safety of emerging pharmacological agents.


In recent years, there has been a significant increase in the number of clinical trials targeting thalamic gliomas. For example, phase II studies are focusing on novel drug combinations aimed at increasing survival rates and improving quality of life for patients. Moreover, many trials are also exploring personalized treatment plans based on genetic profiling, which can allow for tailored therapies that address individual tumor characteristics rather than employing a one-size-fits-all approach.
Key elements of ongoing clinical trials include:
- Monitoring Progress: Tracking patient outcomes to understand the efficacy of new treatments.
- Adaptive Trial Designs: Creating flexible trial structures that can evolve based on interim findings, enhancing the speed and efficiency of research.
- Collaboration: Many trials are now multi-center, leveraging resources and expertise from different institutions to accumulate a larger patient pool.
Evidence from clinical trials not only helps in validating new therapies but also informs regulatory bodies about treatment standards and safety protocols.
By emphasizing these trends in research and clinical trials, we can better understand the evolving landscape of thalamic glioma treatment. Advances in therapeutics and the systematic investigation through clinical trials signal a promising progression towards improved patient management and outcomes.
"Research is essential for unraveling the complexities of thalamic gliomas and developing effective treatment options that could significantly enhance patient care."
Overall, the landscape of thalamic glioma treatment continues to expand, providing hope for more effective management strategies and ultimately, better outcomes for patients.
Long-Term Outcomes and Prognosis
Understanding the long-term outcomes and prognosis of thalamic gliomas is essential for both patients and healthcare providers. The prognosis can significantly influence treatment decisions, which may vary based on individual patient factors such as age, tumor grade, and overall health. Thalamic gliomas are known for their complex nature and challenging location in the brain, making prognostic assessments especially critical. This section will discuss survival rates and quality of life considerations, providing a comprehensive overview of the implications for those affected by this rare form of brain tumor.
Survival Rates
Survival rates for thalamic gliomas are highly variable and can be influenced by several factors. Generally, the prognosis tends to be poorer compared to other types of gliomas due to their infiltrative characteristics and critical location. Research identifies that overall survival rates often fall within a spectrum:
- Low-grade thalamic gliomas may present better survival, with some studies showing five-year survival rates of approximately 50% or more.
- High-grade variants, such as glioblastomas, typically result in considerably lower survival rates, often around 15% at five years.
The age at diagnosis also plays a role. Younger patients often tend to have better outcomes. Historical data show that tumors can be more aggressive in older patients, resulting in decreased survival. Ongoing clinical trials aim to refine these statistics further, incorporating new treatment modalities and their effects on overall survival metrics.
"Survival rates are not just numbers; they are a reflection of individual stories, shaping each patient’s journey through diagnosis, treatment, and beyond."
Quality of Life Considerations
Quality of life is another critical aspect when discussing long-term outcomes. Surviving thalamic gliomas does not solely depend on survival rates; it is equally about the quality of life experienced throughout the treatment and thereafter. Many survivors face various challenges, which can include cognitive impairment, motor function difficulties, and emotional distress.
Key factors influencing quality of life in these patients include:
- Cognitive Function: Cognitive deficits may arise due to the tumor itself or as a side effect of treatment.
- Physical Health: Neurological deficits can impede physical activities, impacting one's ability to perform daily tasks.
- Mental Health: Anxiety and depression are common among those diagnosed with a brain tumor, often requiring complementary mental health support.
To enhance quality of life, rehabilitation strategies should be integrated into treatment plans. Multidisciplinary approaches, involving neurologists, oncologists, psychologists, and rehabilitation specialists, can vastly improve outcomes not just in terms of survival, but also in patient well-being and functionality.
Recognizing the importance of both survival and quality of life is vital in the multifaceted battle against thalamic gliomas. Future research will undoubtedly focus on optimizing treatment to enhance both rate of survival and overall well-being for patients.
Future Directions in Thalamic Glioma Research
Research on thalamic gliomas is evolving rapidly. This part of the article emphasizes how critical it is to redirect scientific inquiry and clinical practice towards more effective treatment approaches and improved patient outcomes. As scientists deepen their understanding of these tumors, innovative methodologies and collaborative efforts become vital. Both approaches aim to address existing challenges that hinder optimal management of thalamic gliomas.
Innovative Treatment Strategies
Currently, treatment for thalamic gliomas remains challenging due to their sophisticated location and complex biology. Innovative treatment strategies are essential. Some of the promising avenues include:
- Targeted Therapy: This involves using drugs that specifically target molecular abnormalities found in thalamic gliomas. By focusing on specific genetic mutations, such as those identified in the K27M mutation, therapies can be more effective.
- Immunotherapy: This approach harnesses the body’s immune system to attack tumor cells. Researchers are exploring ways to enhance these responses, particularly through checkpoint inhibitors.
- Minimally Invasive Surgical Techniques: Advances in neuro-navigation technology enable neurosurgeons to perform resections with greater precision, limiting damage to surrounding healthy tissue.
- Combination Therapies: Employing a regimen that combines various treatment modalities, such as radiation and chemotherapy, can create a synergistic effect, potentially enhancing overall efficacy.
These strategies are currently under investigation in clinical trials, showcasing their relevance in revolutionizing thalamic glioma treatment paradigms.
Collaborative Research Efforts
Collaboration is crucial in the fight against thalamic gliomas. Multiple factor work together across disciplines, institutions, and sectors to elevate standards of care and develop groundbreaking research. Key aspects of collaborative efforts include:
- Multidisciplinary Teams: Specialists from oncology, neurology, radiology, and pathology collaborate to devise comprehensive treatment plans that are tailored to individual patients.
- Inter-Institutional Partnerships: Such partnerships are essential for larger studies that require more extensive patient data. Sharing datasets among institutions can accelerate discoveries and validate findings.
- Public-Private Partnerships: Collaborations between academic institutions and pharmaceutical companies can streamline the translation of research into clinical applications. This partnership also helps in funding innovative research projects that might not have adequate resources otherwise.
- Global Research Networks: Initiatives that encourage global cooperation can help identify trends and share successful protocols worldwide. This knowledge-sharing is indispensable in striking nuances of thalamic gliomas, especially those less understood.
"For advancing treatment options in thalamic gliomas, collaboration is not only beneficial but also necessary."
As researchers address the complexities of these tumors, ongoing collaborative efforts will catalyze innovations and potentially transform patient care. The future of thalamic glioma research rests on these foundations, impacting patient outcomes and informing clinical practice.
This section underscores the imperative to actively pursue fresh avenues in research. It highlights the promise these areas hold for patients affected by thalamic gliomas.
End
In the context of thalamic gliomas, the conclusion serves as a vital synthesis of the various elements discussed throughout this article. Such tumors are indeed rare, yet they represent a significant concern in neurology and oncology due to their complex nature. Having a clear understanding of their pathophysiology, classification, clinical presentations, and treatment modalities is essential not just for specialists, but also for students and researchers in related fields.
Reflecting on the critical aspects covered, it is evident that thalamic gliomas impose unique challenges that extend beyond traditional glioma treatment paradigms. Recognizing both the symptoms and impacts of these tumors clarifies why timely diagnosis is essential. This urgency highlights the need for refining diagnostic techniques and therapeutic approaches, ensuring that patients receive optimal care.
Moreover, ongoing research trends underscore the importance of innovation in treatment strategies. Advances in neuroimaging and genetic profiling open new avenues for targeted therapies, which could potentially improve outcomes for patients affected by these tumors.
The recognition of collaborative efforts within the scientific community also cannot be overlooked. By fostering partnerships between institutions, researchers can accelerate discoveries and enhance the efficacy of treatments. Sharing knowledge across disciplines not only broadens understanding but also enhances the potential for breakthroughs in treatment methodologies.
This conclusion, therefore, is not merely a summary but a call to action for continued investigation and innovation surrounding thalamic gliomas. It is essential to remain committed to understanding these complex tumors. The intersection of clinical practice and research offers a pathway towards better patient outcomes, emphasizing the necessity for an integrated approach in tackling this formidable challenge.
"Patient care is not solely about treating the disease; it is about enhancing life quality through innovative research and dedicated practice."
The synthesis of insights presented here serves as a foundation for future studies and offers a detailed consideration for professionals engaged in this field.







